Abstract
Introduction
Patients with acute myeloid leukemia (AML) that relapse after an allogeneic hematopoietic stem cell transplantation (HSCT) have a dismal prognosis. However, especially with novel salvage options available, a second allogeneic HSCT (2nd HSCT) may still have curative potential in selected patients. Identifying factors that influence outcomes in AML patients undergoing a 2nd HSCT is important to improve outcomes and inform treatment decisions. Here we analyzed AML patients that received a 2nd HSCT after disease relapse.
Methods
We identified and analyzed 57 patients (33 male) with AML who received a 2nd allogeneic HSCT at our center between 2000 and 2022. Median age at 2nd HSCT was 55.5 years (range 25.2-74.9). The primary diagnosis before the 1st HSCT was AML (n=41), myelodysplastic neoplasm (MDS, n=10), or myeloproliferative neoplasm (MPN), or MDS/MPN (n=6). All patients relapsed with an AML after 1st HSCT. European LeukemiaNet Risk (ELN) 2022 genetic risk at AML diagnosis was evaluable for 40 patients and was in 20% favorable, in 25% intermediate, and in 55% adverse. At 2nd HSCT, 37 patients (65%) were in complete remission with or without peripheral recovery (CR/CRi) or in morphologic leukemia-free state (MLFS), while 20 (35%) had active disease. Conditioning intensity according to EBMT criteria were non-myeloablative (n=33, 58%) or reduced intensity (n=24, 42%). In all but one patient, a new donor for the 2nd HSCT was selected. Donors for 2nd HSCT were matched related (4%), matched unrelated (61%), mismatched unrelated (32%), or haploidentical (4%). Median time from 1st HSCT to 2nd HSCT was 671 days (range 83-5218). Median follow up after 2nd HSCT for patients alive was 2.0 years.
Results
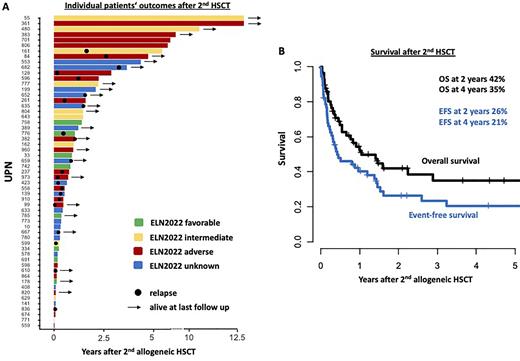
Of all analyzed patients 22 (39%) relapsed and 23 patients (40%) died in remission (Figure 1A). Two and 4 years after 2nd HSCT, event-free survival (EFS) was 26% and 21%, respectively, and overall survival (OS) was 42% and 35% (Figure 1B), respectively. In the whole cohort, patients who received their 1st HSCT for a MDS/MPN or MPN and the 2nd HSCT after disease progression to AML had a significantly shorter EFS (P=.002) and by trend a shorter OS (P=.08), compared to patients transplanted for AML after MDS or AML relapse. Early relapse after 1st HSCT (<1 year) tended to be associated with a shorter EFS (P=.10) and a shorter OS (P=.04). Development of a chronic graft-versus-host disease (GvHD) after 2nd HSCT (implicating an associated graft-versus-leukemia effect) associated with longer EFS (P=.005) and OS (P=.05). In contrast, none of the following variables: ELN2022 genetic risk at diagnosis (EFS P=.30, OS P=.20), age at 2nd HSCT (> or ≤ 60 years, EFS P=.40, OS P=.80), remission status at 2nd HSCT (CR/CRi/MLFS vs active disease, EFS P=.90, OS P=.80), the chosen conditioning intensity (NMA vs RIC, EFS P=.80, OS P=.50), or the kind of donor (matched vs mismatched, EFS P=.30, OS P=.20), impacted OS after 2nd HSCT. In multivariate analyses, prior HSCT for MDS/MPN or MPN (Odds Ratio [OR] 5.74, P=.04) and absence of a chronic GvHD (OR 0.52, P=.01) associated with shorter EFS, while early relapse after 1st HSCT (<1 year, OR 0.28, P=.05) and absence of a chronic GvHD (HR 0.33, P=.02) associated with shorter OS after a 2nd HSCT.
Conclusion
For patients who experience an AML relapse of their myeloid disease after HSCT, performing a 2nd HSCT after reduced intensity or non-myeloablative conditioning may be a feasible and potentially curative treatment option, with an OS of 35% after 4 years. Irrespective of diagnostic ELN2022 genetic risk, a longer remission after 1st HSCT and presence of chronic GvHD associated with improved survival following a 2nd HSCT.
Disclosures
Herling:Takeda: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Janpix: Honoraria, Research Funding; EDO-Mundipharma: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Metzeler:Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; AbbVie: Honoraria; Curis: Research Funding. Merz:BMS Celgene: Honoraria; Janssen: Honoraria. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Platzbecker:Janssen: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; Geron: Honoraria; BMS/Celgene: Honoraria; Novartis: Honoraria; Silence Therapeutics: Honoraria; Jazz: Honoraria. Schwind:Novartis: Honoraria. Jentzsch:Jazz Pharmaceuticals: Honoraria; Novartis: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.